UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
ALKERMES PUBLIC LIMITED COMPANY
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
||
|
(State or other jurisdiction |
|
(Commission |
|
(IRS Employer |
||
|
of incorporation) |
|
File Number) |
|
Identification No.) |
||
|
|
|
|
||||
|
|
|
|
||||
|
|
||||||
|
|
||||||
|
(Address of principal executive offices) |
||||||
Registrant's telephone number, including area code: +
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
From January 13-15, 2020, Alkermes plc (the “Company”) will participate in investor activities at the J.P. Morgan Healthcare Conference. A copy of the Company’s corporate presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Form 8-K”) and is incorporated in this Item 7.01 by reference.
The information contained in this Form 8-K, including in this Item 7.01, and in Exhibit 99.1 furnished herewith, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit |
|
|
|
No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
ALKERMES PLC |
||
|
|
|
||
|
Date: January 13, 2020 |
By: |
|
/s/ David J. Gaffin |
|
|
|
|
David J. Gaffin |
|
|
|
|
Senior Vice President, Chief Legal Officer, Chief Compliance Officer and Secretary |
3

Shaping the Future of the Business Richard Pops Chief Executive Officer Exhibit 99.1 38th Annual J.P. Morgan Healthcare Conference January 15, 2020

Forward-Looking Statements Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the company’s mission of advancing patient-centered care and redefining what constitutes ‘successful treatment’; the company’s evolving research and development capabilities and focus, including the therapeutic areas that the company may pursue; the company’s expectations with respect to its current and future financial and operating performance, business plans or prospects, including expectations relating to potential expansion of the company’s product portfolio, continued growth of revenue from the company’s commercial products, new potential elements of revenue, including royalty and manufacturing revenues for VUMERITY® and potential revenue from ALKS 3831 if approved, and the company’s potential to deliver profitability; the real-world impact and potential therapeutic and commercial value of the company’s marketed and development products; timelines, plans and expectations for development activities relating to the company’s products and product development candidates in both central nervous system (“CNS”) disorders and oncology, including ongoing enrollment and other progress across the ARTISTRY clinical development program for ALKS 4230 and emerging data from such program, and lead optimization and/or IND-enabling activities for the company’s preclinical compounds, including the company’s HDAC inhibitor platform and the company’s IL-10 fusion protein platform; the company’s expectations relating to regulatory actions by the U.S. Food and Drug Administration (“FDA”) relating to the company’s new drug application (“NDA”) submission for ALKS 3831, including the adequacy of the data contained in the NDA to serve as the basis of approval of ALKS 3831 for both the treatment of schizophrenia and the treatment of bipolar I disorder; the company’s growing commercial infrastructure and expectations concerning commercial activities relating to the company’s products and product candidates, including preparations for the potential commercial launch of ALKS 3831; and the company’s expectations regarding the duration of patent protection for VUMERITY. The company cautions that forward-looking statements are inherently uncertain. Although the company believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, the forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation and other patent litigation, related to any of the company’s products may lead to competition from generic drug manufacturers; data from clinical trials may be interpreted by the FDA in different ways than the company interprets it; the FDA may not agree with the company’s regulatory approval strategies or components of the company’s filings for its products, including its clinical trial designs, conduct and methodologies or the sufficiency of the results thereof to support approval; the company’s development activities may not be completed on time or at all; the results of the company’s development activities may not be positive, or predictive of real-world results or of results in subsequent trials, and preliminary or interim results of the company’s development activities may not be predictive of final results of such activities, results of future preclinical or clinical trials or real-world results; the company and its licensees may not be able to continue to successfully commercialize their products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the FDA or regulatory authorities outside the U.S. may make adverse decisions regarding the company’s products; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s most recent Annual Report on Form 10-K and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov and on the company’s website at www.alkermes.com in the “Investors—SEC filings” section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Note Regarding Trademarks: The company and its affiliates are the owners of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, ARISTADA INITIO®, and VIVITROL®. VUMERITY® is a registered trademark of Biogen MA Inc., used by Alkermes under license. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto.

Alkermes’ Distinctive Mission and Impact Through our advocacy, we advance patient-centered care and seek to redefine what constitutes successful treatment. Our science and medicines are making a real-world impact in the treatment of serious diseases.

Advancing a diversified CNS and oncology pipeline Positioning the business to deliver long-term growth and profitability Expanding and driving growth of our product portfolio Actively Shaping the Future of the Business

Expanding and Driving Growth of Our Product Portfolio

Proprietary Commercial Products for Addiction and Schizophrenia Extended-release opioid antagonist provides therapeutic levels of naltrexone for a one-month period Only medication indicated for the prevention of relapse to opioid dependence, following opioid detoxification; Indicated for the treatment of alcohol dependence *ARISTADA INITIO + single 30 mg oral dose of aripiprazole replaces need for concomitant three weeks of oral aripiprazole for initiation of ARISTADA, with relevant levels of aripiprazole concentration reached within four days. The first ARISTADA dose may be administered on the same day as ARISTADA INITIO or up to 10 days thereafter. Long-acting injectable (LAI) atypical antipsychotic indicated for the treatment of schizophrenia First and only LAI with ability to fully dose on day one* for up to two months with ARISTADA INITIO® regimen

$1 Billion Topline Commercial Enterprise Driven by Growth of Proprietary Products Proprietary Product Net Sales ($M) 2019 Growing proprietary commercial products VIVITROL® ARISTADA® Financial foundation of license, royalty and manufacturing revenues New potential revenue elements: 2020 Royalty and manufacturing: VUMERITY® 2021 Proprietary psychiatry portfolio: Potential launch of ALKS 3831 (pending FDA approval)

VUMERITY® (Diroximel Fumarate) for Multiple Sclerosis (MS) Novel oral fumarate with a distinct chemical structure for the treatment of relapsing forms of MS Discovered and developed by Alkermes Approved by FDA in October 2019 Exclusive, worldwide license to commercialize held by Biogen Launched by Biogen in late Q4 2019 Composition of matter patent extends into 2033 ~325K patients treated for multiple sclerosis in the U.S. (~75% RRMS*)1 15K MS patients new to therapy each year 60K MS patients change therapy each year Now Approved *RRMS: Relapsing Remitting Multiple Sclerosis Decision Resources MS Disease Landscape (Nov. 2016)

ALKS 3831: A Potential New Oral Treatment for Adults With Schizophrenia and Adults With Bipolar I Disorder Investigational antipsychotic designed to offer robust efficacy of olanzapine; addition of samidorphan intended to mitigate olanzapine-associated weight gain Single NDA for treatment of adults with schizophrenia and adults with bipolar I disorder submitted Nov. 2019 Conducted pre-NDA meeting to discuss contents of NDA and FDA requirements Fixed-dose combination Bilayer tablet of samidorphan (10 mg) and olanzapine (5 mg, 10 mg, 15 mg, or 20 mg) ALKS 3831 Launch Preparations Disease State Awareness Payer Engagement Building on existing commercial infrastructure and capabilities in schizophrenia

Olanzapine: Atypical Antipsychotic Treatment Driven by Efficacy Source: IQVIA R12M as of Nov. 2019 Branded orals include: LATUDA®, REXULTI®, VRAYLAR®, SAPHRIS®, FANAPT®

Advancing a Diversified Pipeline CNS and Oncology

Alkermes’ Evolving Research and Development Capabilities 2000s 2010s 2020 Formulation & Drug Delivery Modifying Existing Small Molecules, Proteins, Peptides LAI Atypical Antipsychotics VIVITROL® BYDUREON® New Molecular Entities Prodrug & NCE Chemistry, Cytokine Engineering ARISTADA® VUMERITY® ALKS 3831 ALKS 4230 New Biology in CNS & Oncology Small Molecule Chemistry, Protein Fusion Psychiatry Neurodegeneration Oncology R&D Focus Capabilities Approved Medicines, Drug Candidates & Therapeutic Areas

Building Fully-Integrated Capabilities Across CNS and Oncology CNS Research & Discovery ü Formulation & CMC ü Clinical Trial Operations ü Regulatory Affairs ü Medical Affairs ü Commercial ü Patient Engagement ü Expanding ex-U.S. trial network Evaluating strategic development and commercial partnerships Oncology ü ü ü ü ü ü

Preclinical Phase 1 Phase 2 Phase 3 NDA ALKS 3831 Schizophrenia/ Bipolar I Disorder* ALKS 4230 Immuno-oncology (Intravenous Dosing) Immuno-oncology (Subcutaneous Dosing) IL-10 Fusion Proteins Immuno-oncology Selective HDAC Inhibitors Neurodegenerative Disorders (Orphan) Neurodegenerative Disorders (Prevalent, Non-orphan) Oncology Research and Development Pipeline: Novel Molecules in High-Potential Therapeutic Areas Oncology CNS *NDA submitted to FDA in Nov. 2019

Advancing a Diversified Pipeline Cytokine Platform

ALKS 4230: Selective IL-2 Fusion Protein Novel investigational drug designed to leverage proven anti-tumor effects of interleukin-2 (IL-2) pathway Stable, single polypeptide designed to selectively bind to intermediate-affinity IL-2 receptor and expand tumor-killing CD8+ and Natural Killer (NK) T cells, and have negligible effects on Treg expansion ARTISTRY-1 and ARTISTRY-2 phase 1/2 studies ongoing Data presented at Society of Immunotherapy of Cancer meeting in Nov. 2019 ALKS 4230

IL-2 Activates and Expands Immune Suppressive Regulatory T Cells That Dampen Anti-Cancer Immune Responses Graphics for illustrative purposes only. b g Intermediate-Affinity Receptor-bearing Cell CD8+ T cell NK cell CD122 CD132 b g a High-Affinity Receptor-bearing Cell Treg cell CD25 CD122 CD132 Fight Cancer CD8+ T cells NK cells Suppress Immune Response Treg cells IL-2 IL-2

ALKS 4230 Designed to Selectively Activate Intermediate-Affinity Receptor Graphics for illustrative purposes only. ALKS 4230 Design Intention: Preferentially expand cancer-fighting CD8+ T cells and NK cells to potentially improve anti-tumor efficacy Prevent IL-2-derived expansion of Treg cells to minimize inhibition of immune response Mitigate certain side effects of IL-2, including vascular leak syndrome b g Intermediate-Affinity Receptor-bearing Cell CD8+ T cell NK cell Fight Cancer CD8+ T cells NK cells CD122 CD132 ALKS 4230 ALKS 4230 ALKS 4230 b g a High-Affinity Receptor-bearing Cell Treg cell CD25 CD122 CD132

Overview of ALKS 4230 Clinical Development Program ARTISTRY-1 Phase 1/2 ARTISTRY-2 Phase 1/2 6-week monotherapy lead-in phase Followed by ALKS 4230 + pembrolizumab combination Evaluating once-weekly and once every three weeks dosing Efficacy expansion phase planned Part A: Monotherapy dose escalation Part B: Monotherapy dose expansion Part C: ALKS 4230 + pembrolizumab combination ION-01 Phase 2 Collaboration with Fred Hutchinson Cancer Research Center ALKS 4230 + pembrolizumab combination Assessment of tumor microenvironment from paired biopsies Predictive biomarker assessments Intravenous dosing Refractory advanced solid tumors Subcutaneous dosing Refractory advanced solid tumors Intravenous dosing Anti-PD-1 pre-treated HNSCC* patients HNSCC*: Head and neck squamous cell carcinoma

Interleukin-10: Concentration-Dependent, Immuno-Regulatory Cytokine Low concentrations Higher concentrations Tumor-associated macrophage CD8+ T cell CD4+ T cell NK cell B cell Dendritic cell Anti-inflammatory/ Immunosuppressive properties Anti-tumor cytotoxic activity Concentration and dose threshold IL-10 Low concentrations: Activates innate immune cells to provide anti-inflammatory and immunosuppressive activities Higher concentrations: Activates anti-tumor CD8+ T cells and Natural Killer cells Short half-life of native IL-10 limits ability to achieve and maintain higher concentrations Mannino MH et al. Cancer Lett. 2015.

Expanding Investigational Cytokine Therapy Portfolio: IL-10 Fusion Proteins Alkermes’ design objectives: Antibody scaffolding to extend circulating half-life Achieve higher concentrations Reduce dosing frequency Retain anti-tumor functions of IL-10 Fc-mediated activities to provide potential additional effects Lead evaluation underway Protein engineering expertise yields differentiated molecular design CH1 CL IL-10 IL-10 CH1 CL IL-10 IL-10 Fc
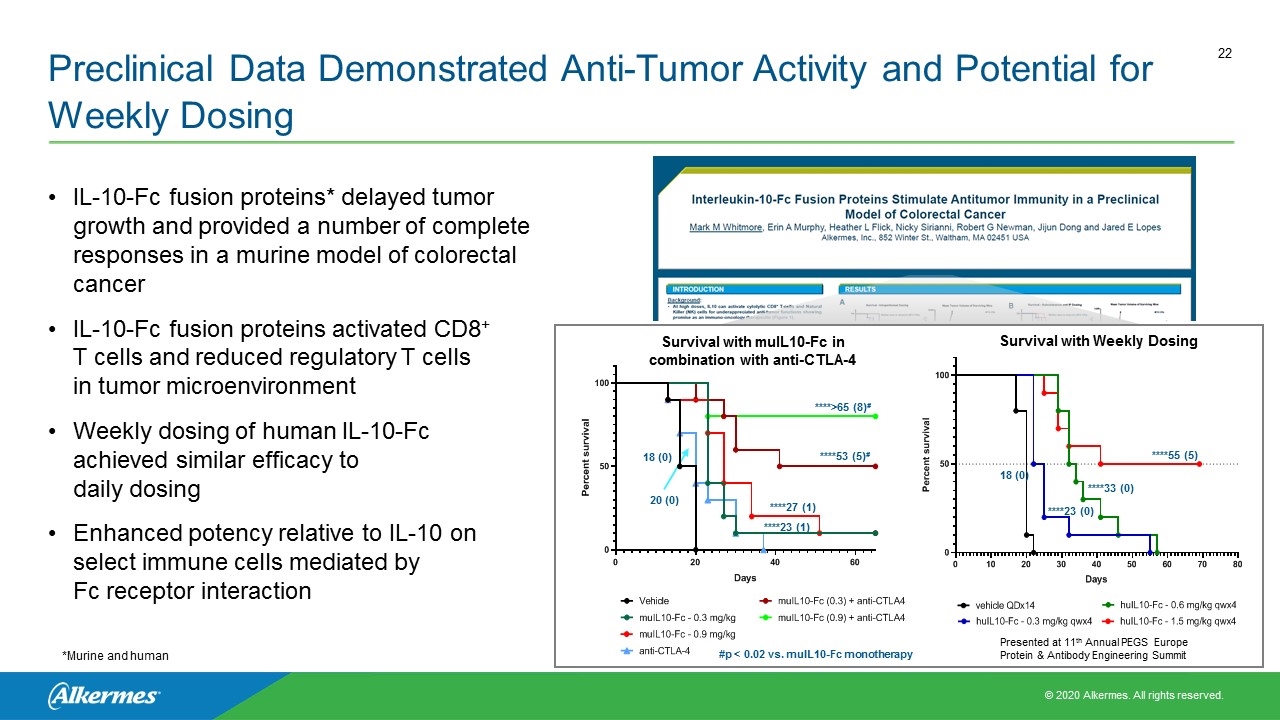
Preclinical Data Demonstrated Anti-Tumor Activity and Potential for Weekly Dosing IL-10-Fc fusion proteins* delayed tumor growth and provided a number of complete responses in a murine model of colorectal cancer IL-10-Fc fusion proteins activated CD8+ T cells and reduced regulatory T cells in tumor microenvironment Weekly dosing of human IL-10-Fc achieved similar efficacy to daily dosing Enhanced potency relative to IL-10 on select immune cells mediated by Fc receptor interaction *Murine and human Presented at 11th Annual PEGS Europe Protein & Antibody Engineering Summit 18 (0) ****23 (0) ****33 (0) ****55 (5) Survival with Weekly Dosing ****>65 (8)# ****53 (5)# 20 (0) 18 (0) ****27 (1) ****23 (1) #p < 0.02 vs. muIL10-Fc monotherapy Survival with muIL10-Fc in combination with anti-CTLA-4

Advancing a Diversified Pipeline Selective HDAC Inhibitors

Synapses Play a Vital Role in Brain Function Axon Dendritic Spine Pre-synaptic vesicle Neurotransmitter released into synapse Post-synaptic receptor Synapses are the points of communication within the network of neurons that make up the brain Synaptic function is critical to brain development, learning and memory Synaptogenesis is the formation of synapses between neurons in the nervous system that occurs throughout a healthy person's lifespan

Synaptopathies Span Multiple Neurological Diseases Independent of Underlying Pathology Synaptic loss and dysfunction occur across a wide range of disorders and are associated with clinical symptoms1 Increased synaptic density and function strengthen neuronal connectivity, potentially leading to clinically relevant benefits2, independent of underlying disease pathology Neuropsychiatric Neurodegenerative Neurodevelopment Bipolar spectrum disorder Frontotemporal dementia Parkinson’s Autism spectrum disorder Schizophrenia Huntington’s Cochlear Fragile X syndrome Major depressive disorder Alzheimer’s Retinal Epilepsy 1Lepeta, K et al. J Neurochem. 2016. 2Verstraelen, P et al. Front. Neurosci. 2018.

Loss of Synapses Correlated to Cognitive Decline in Neurodegenerative Patients Preserved synaptic density observed in cognitively normal patients with underlying Alzheimer’s Disease pathology (CAD) Spine density was similar among control and CAD cases but was reduced significantly in patients with Alzheimer’s Disease (AD) that demonstrated clinical dementia Boros et al. Annals of Neurology. 2017. Masliah E. et al. J Alzheimers Dis. 2001. Synapse loss tracked disease progression ~25% ~35% ~45% Disease severity Synapse loss * * * Control 9 Mild 9 Moderate 8 Severe 16 N= 0 50 150 100 Control Cognitively normal patient with underlying AD pathology AD patient that demonstrated clinical dementia

Epigenetic Control of Synaptogenesis Acetylation of histones increases accessibility of DNA for transcription of multiple genes associated with synaptogenesis Deacetylation of histones by HDACs (histone deacetylase) causes tight coiling of DNA and closed chromatin leading to gene repression Brain-penetrant HDACs increase acetylation, driving prosynaptic gene expression and ultimately synaptogenesis Histone DNA accessible, gene active Closed chromatin Open chromatin DNA inaccessible, gene inactive Acetyl group Histone tail

Extensive Literature Discussing Prosynaptic Effects of HDAC Inhibitors Modulation of long-term memory for object recognition via HDAC inhibition Crebinostat: A Novel Cognitive Enhancer that Inhibits Histone Deacetylase Activity and Modulates Chomatin-Mediated Neuroplasticity Molecular Gene and/or protein modification Structural Synapse formation Functional Increased long-term potentiation

New Chemistry Targets Selective HDAC Complexes Approved HDAC inhibitor compounds have been limited by hematological toxicities, precluding application to chronic neurologic conditions HDACs function in association with multi-protein complexes which determine their activity Alkermes’ proprietary compounds target specific subsets of HDAC complexes CoREST (co-repressor of repressor element-1 silencing transcription factor) is directly involved in repression of prosynaptic genes in neuronal tissue HDAC Complexes CoREST Sin3 Adapted from Seto & Yoshida. Cold Spring Harb Perspect Biol. 2014 p110 Sox-like CoREST p80 HDAC2 HDAC1 NuRD MBD3 Mi2 MTA2 HDAC2 RbAP46 RbAP48 HDAC1 RbAP46 RbAP48 Sap18 Sap30 Sin3 HDAC2 HDAC1

Toxicity CoREST Selectivity Brain Penetration Progress Across Three Key Areas of Optimization of HDAC Inhibitors for Synaptopathies Demonstrated significantly improved hematological safety* Selective HDAC-CoREST modulation* activity observed ü ü ü *Fuller et al. ACS Chem. Neurosci. 2019, 10, 1729-1743 Novel chemotypes designed to enable good brain PK and ADME properties PK: Pharmacokinetic; ADME: Absorption, distribution, metabolism and excretion

CoREST Complex-Selective HDAC Inhibitors Showed Prosynaptic Effects and Improved Safety Profile “…novel HDAC inhibitor compounds that selectively inhibit the HDAC−co-repressor of repressor element-1 silencing transcription factor (CoREST) complex while minimizing hematological side effects…selectively targeting the CoREST co-repressor complex…results in increased spine density and synaptic proteins, and improved long-term potentiation in a mouse model at doses that provide a substantial safety margin that would enable chronic treatment.” Fuller et al. CoREST Complex-Selective Histone Deacetylase Inhibitors Show Prosynaptic Effects and an Improved Safety Profile To Enable Treatment of Synaptopathies. ACS Chem. Neurosci. 2019, 10, 3, 1729-1743.

Advancing Preclinical Research and IND-Enabling Activities HDAC CoREST inhibitors for synaptopathies Pursue IND-enabling activities for lead preclinical compounds Potential utility across highly-prevalent neurodegenerative diseases such as Alzheimer’s Disease as well as orphan diseases such as frontotemporal dementia and Huntington’s Disease Oncology and other disease areas Continue exploratory work to assess the potential utility of selective HDAC modulation Translational development and biomarkers Continue development of biomarker and translational tools to help demonstrate potential target engagement and efficacy

Positioning the Business to Deliver Long-Term Growth and Profitability

Positioning Alkermes for Long-Term Growth Revenue Growth Pipeline Governance Capital Allocation Cost Structure

Positioning Alkermes for Long-Term Growth VIVITROL® & ARISTADA® : Executing commercial plans VUMERITY®: Received FDA approval; Launched by Biogen ALKS 3831: Submitted NDA and preparing for launch Revenue Growth Governance Pipeline Cost Structure Capital Allocation

Positioning Alkermes for Long-Term Growth Revenue Growth Governance Pipeline Cost Structure Capital Allocation VIVITROL® & ARISTADA® : Executing commercial plans VUMERITY®: Received FDA approval; Launched by Biogen ALKS 3831: Submitted NDA and preparing for launch Expanded ALKS 4230 program driven by emerging data Introduced IL-10-Fc program and HDAC inhibitor platform

Positioning Alkermes for Long-Term Growth Revenue Growth Governance Pipeline Cost Structure Capital Allocation VIVITROL® & ARISTADA® : Executing commercial plans VUMERITY®: Received FDA approval; Launched by Biogen ALKS 3831: Submitted NDA and preparing for launch Expanded ALKS 4230 program driven by emerging data Introduced IL-10-Fc program and HDAC inhibitor platform Acquired Rodin Therapeutics Focusing investment in highest-potential R&D programs

Positioning Alkermes for Long-Term Growth Revenue Growth Governance Pipeline Cost Structure Capital Allocation VIVITROL® & ARISTADA® : Executing commercial plans VUMERITY®: Received FDA approval; Launched by Biogen ALKS 3831: Submitted NDA and preparing for launch Expanded ALKS 4230 program driven by emerging data Introduced IL-10-Fc program and HDAC inhibitor platform Acquired Rodin Therapeutics Focusing investment in highest-potential R&D programs Implemented strategic restructuring to reduce cost structure Accelerated toward sustained non-GAAP profitability

Positioning Alkermes for Long-Term Growth Revenue Growth Governance Pipeline Cost Structure Capital Allocation VIVITROL® & ARISTADA® : Executing commercial plans VUMERITY®: Received FDA approval; Launched by Biogen ALKS 3831: Submitted NDA and preparing for launch Expanded ALKS 4230 program driven by emerging data Introduced IL-10-Fc program and HDAC inhibitor platform Acquired Rodin Therapeutics Focusing investment in highest-potential R&D programs Implemented strategic restructuring to reduce cost structure Accelerated sustained non-GAAP profitability Added expertise in oncology and strategic value creation to Board with appointment of two new Directors

Positioning Alkermes for Long-Term Growth Revenue Growth Governance Pipeline Cost Structure Capital Allocation VIVITROL® & ARISTADA®: Executing commercial plans VUMERITY®: Received FDA approval; Launched by Biogen ALKS 3831: Submitted NDA and preparing for launch Expanded ALKS 4230 program driven by emerging data Introduced IL-10-Fc program and HDAC inhibitor platform Acquired Rodin Therapeutics Focusing investment in highest-potential R&D programs Implemented strategic restructuring to reduce cost structure Accelerated sustained non-GAAP profitability Added expertise in oncology and strategic value creation to Board with appointment of two new Directors

Advancing a diversified CNS and oncology pipeline Positioning the business to deliver long-term growth and profitability Expanding and driving growth of our product portfolio Actively Shaping the Future of the Business

www.alkermes.com
