UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
ALKERMES PUBLIC LIMITED COMPANY
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
||
|
(State or other jurisdiction |
|
(Commission |
|
(IRS Employer |
||
|
of incorporation) |
|
File Number) |
|
Identification No.) |
||
|
|
|
|
||||
|
|
|
|
||||
|
|
||||||
|
|
||||||
|
(Address of principal executive offices) |
||||||
Registrant's telephone number, including area code: +
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
From January 11-14, 2021, Alkermes plc (the “Company”) will participate in investor activities as part of the virtual J.P. Morgan Healthcare Conference. A copy of the Company’s corporate presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated in this Item 7.01 by reference.
The information in this Item 7.01, and in Exhibit 99.1 furnished herewith, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit |
|
|
|
No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
ALKERMES PLC |
||
|
|
|
||
|
Date: January 11, 2021 |
By: |
|
/s/ David J. Gaffin |
|
|
|
|
David J. Gaffin |
|
|
|
|
Senior Vice President, Chief Legal Officer, Chief Compliance Officer and Secretary |
3

Focus on Value Creation Richard Pops Chief Executive Officer 39th Annual J.P. Morgan Healthcare Conference January 13, 2021 Exhibit 99.1

Forward-Looking Statements Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the company’s mission of, and impact in, advancing cutting-edge science, developing innovative medicines, and addressing critical public health challenges; the company’s expectations with respect to its current and future financial and operating performance, business plans or prospects, including potential growth of revenue from its commercial products, potential diversification of its product portfolio, therapeutic areas that the company may pursue and the company’s plans to manage for growth and long-term profitability through execution of its Value Enhancement Plan, including its commitment to profitability targets, optimization of its cost structure and exploration of strategic opportunities; the potential therapeutic and commercial value of the company’s marketed and development products; timelines, plans and expectations for development activities relating to the company’s products and product development candidates in both neuroscience and oncology, including (i) for nemvaleukin alfa (“nemvaleukin”), plans to initiate additional studies with IV nemvaleukin, select additional tumor types to pursue, and explore strategic collaborations and (ii) for ALKS 1140, plans to begin phase 1 first-in-human trials; the company’s expectations relating to regulatory activities and interactions, including the U.S. Food and Drug Administration’s (“FDA”) PDUFA target action date for the company’s new drug application (“NDA”) for ALKS 3831 and plans to advance discussions on registration plans for nemvaleukin with regulatory agencies; expectations concerning commercial activities relating to the company’s products and product candidates, including preparations for the potential commercial launch of ALKS 3831 and the ability of the company’s commercial model to drive growth and competitive advantage. The company cautions that forward-looking statements are inherently uncertain. These risks, assumptions and uncertainties include, among others: the potential impacts of the COVID-19 pandemic and efforts to mitigate its spread on the company’s business, results of operations and financial condition; the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation and other patent litigation, related to any of the company’s products may lead to competition from generic drug manufacturers; data from clinical trials may be interpreted by the FDA in different ways than the company interprets it; the FDA may not agree with the company’s regulatory approval strategies or components of the company’s NDAs, including clinical trial designs, conduct and methodologies, manufacturing processes and facilities, or the adequacy of the data included in the NDAs to support the FDA’s requirements for approval; the FDA or regulatory authorities outside the U.S. may make adverse decisions regarding the company’s products, including with respect to the NDA for ALKS 3831; the company’s development activities may not be completed on time or at all; the results of the company’s development activities may not be positive, or predictive of real-world results or of results in subsequent trials, and preliminary or interim results of the company’s development activities may not be predictive of final results of such activities, results of future preclinical or clinical trials or real-world results; the company and its licensees may not be able to successfully commercialize their products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended Dec. 31, 2019, the company’s quarterly report on Form 10-Q for the quarter ended June 30, 2020 and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov and on the company’s website at www.alkermes.com in the “Investors—SEC filings” section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Note Regarding Trademarks: The company and its affiliates are the owners of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, ARISTADA INITIO®, VIVITROL®, and LYBALVITM. VUMERITY® is a registered trademark of Biogen MA Inc., used by Alkermes under license. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto.

Alkermes’ Distinctive Mission 3 Advance cutting-edge science, develop innovative medicines, and engage in patient-focused advocacy to address critical public health challenges

Execution Against Our Strategic Priorities Commercial Execution VIVITROL® and ARISTADA® Strong performance in a complex environment Adapted commercial strategy in response to COVID-19 Prepared for synergistic launch of ALKS 3831 within psychiatry portfolio Supported launch of VUMERITY® Advancement of Highest Potential R&D Programs Completed successful Advisory Committee meeting for ALKS 3831* Advanced nemvaleukin alfa (ALKS 4230) development program Observed anti-tumor activity in monotherapy and combination settings with intravenous administration Accelerated patient enrollment and expanded clinical trial network globally Nominated first clinical candidate from HDAC** inhibitor program Efficient Management of Operating Structure and Strong Governance Adapted cost structure in response to COVID-19-related disruptions Announced Value Enhancement Plan Commitment to profitability targets Focus on strategic opportunities Continued Board refreshment Appointed two new independent directors Announced upcoming retirement of two long-serving directors 2020 Key Accomplishments *NDA resubmission under review following FDA Complete Response Letter and records requests relating to manufacturing of ALKS 3831. **HDAC: histone deacetylase

Focus on Value Creation in 2021: Three Key Components Grow and Diversify Commercial Revenues Demonstrate Value of R&D Investments Manage for Growth & Long-Term Profitability Drive VIVITROL® and ARISTADA® net sales Support VUMERITY® growth Launch ALKS 3831 (PDUFA* June 1, 2021) Nemvaleukin alfa Determine registration pathway Demonstrate anti-tumor activity Explore strategic collaboration ALKS 1140 (CoREST-selective HDAC inhibitor) Initiate phase 1/FIH study Investor Day Provide update on pipeline platforms and programs Operationalize commitment to profitability targets Optimize cost structure and drive operating leverage Explore strategic opportunities to maximize value and enhance profitability 1 2 3 *Prescription Drug User Fee Act
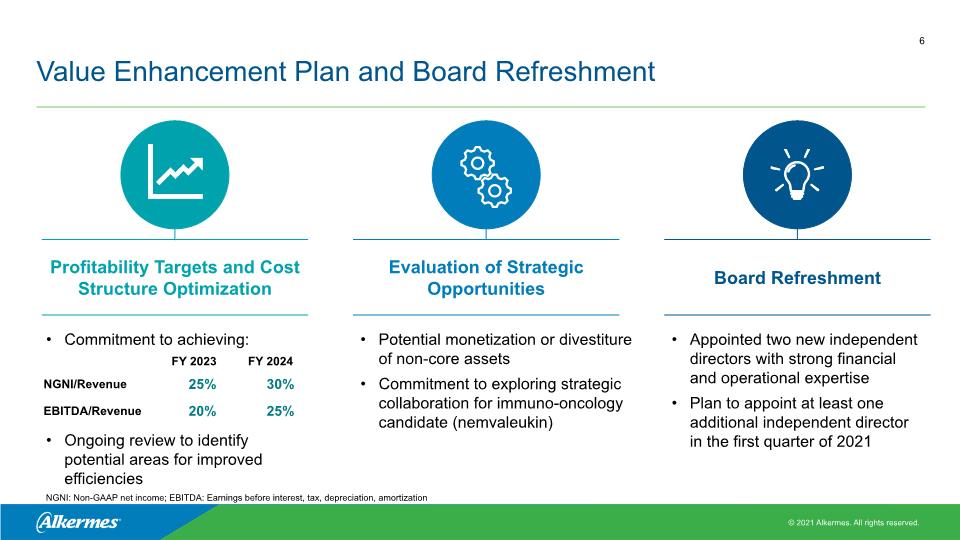
Value Enhancement Plan and Board Refreshment Profitability Targets and Cost Structure Optimization Evaluation of Strategic Opportunities Board Refreshment Commitment to achieving: Ongoing review to identify potential areas for improved efficiencies Potential monetization or divestiture of non-core assets Commitment to exploring strategic collaboration for immuno-oncology candidate (nemvaleukin) Appointed two new independent directors with strong financial and operational expertise Plan to appoint at least one additional independent director in the first quarter of 2021 NGNI: Non-GAAP net income; EBITDA: Earnings before interest, tax, depreciation, amortization

Alkermes Commercial Model: Designed to Drive Growth and Competitive Advantage Dynamic Market Environment Alkermes Commercial Model Flexible and scalable Allows for rapid adjustment to changes in healthcare landscape Strong field presence supplemented by digital capabilities & non-personal promotion Interactions with Federal and State policymakers Increased use of telepsychiatry** Restricted access to healthcare providers** Growing strength of government payers Focus on pharmacoeconomic value *Pending FDA approval **Impacted by COVID-19 ALKS 3831*

© 2021 Alkermes. All rights reserved.

VIVITROL®: Distinctive Product for a Major Public Health Need Extended-release opioid antagonist provides therapeutic levels of naltrexone for a one-month period Indicated for the treatment of alcohol dependence Indicated for the prevention of relapse to opioid dependence, following opioid detoxification Full prescribing information for VIVITROL may be found at www.vivitrol.com

VIVITROL Net Sales ($M) VIVITROL®: Stabilization and Recovery Following COVID-19 Related Disruption VIVITROL Units (Four-week Average) COVID-19 disruptions began VIVITROL stabilization and recovery began *TTM (trailing 12 month) data includes Q4’19 through Q3’20 VIVITROL net sales.

VIVITROL®: 2021 Focus Opioid Dependence (OD) Alcohol Dependence (AD) Focus on select settings of care to increase use of VIVITROL for the prevention of relapse to opioid dependence, following detoxification Accelerate VIVITROL adoption in alcohol dependence by building on the growing use of medications ~14.5M 14% ~1.6M 90% of diagnosed AUD patients treated with MAT in 2019* people living with alcohol use disorder (AUD)* people living with opioid use disorder (OUD)* of diagnosed OUD patients treated with MAT in 2019* *2019 SAMHSA National Survey of Drug Use and Health. AD and OD (DSM-IV) (indications for VIVITROL) approximate moderate or severe AUD and OUD (DSM 5), respectively. Compton WM et al. Drug Alcohol Depend. 2013;132(1-2):387-390. Full prescribing information for VIVITROL may be found at www.vivitrol.com

© 2021 Alkermes. All rights reserved.

ARISTADA®: LAI for Schizophrenia Suited for Today’s Environment Long-acting injectable (LAI) atypical antipsychotic indicated for the treatment of schizophrenia Novel molecular entity designed to address the real-world needs of patients and providers in the community Full prescribing information for ARISTADA may be found at www.aristada.com

ARISTADA® TRx MOT Growth Outpaced the Atypical LAI Market ARISTADA TRx MOT Growth Rate TRx Data: IQVIA NPA data Nov R3 MOT: Months of therapy *TTM (trailing 12 month) data includes Q4’19 through Q3’20 ARISTADA net sales results. ARISTADA Net Sales ($M)

ARISTADA®: A Unique Product Offering ARISTADA INITIO® Regimen* ARISTADA 2-Month Dose Only long-acting treatment for schizophrenia that offers: Single-day initiation An option for dosing only six times a year *ARISTADA INITIO + single 30 mg oral dose of aripiprazole replaces need for concomitant three weeks of oral aripiprazole for initiation of ARISTADA, with relevant levels of aripiprazole concentration reached within four days. The first ARISTADA dose may be administered on the same day as ARISTADA INITIO or up to 10 days thereafter.
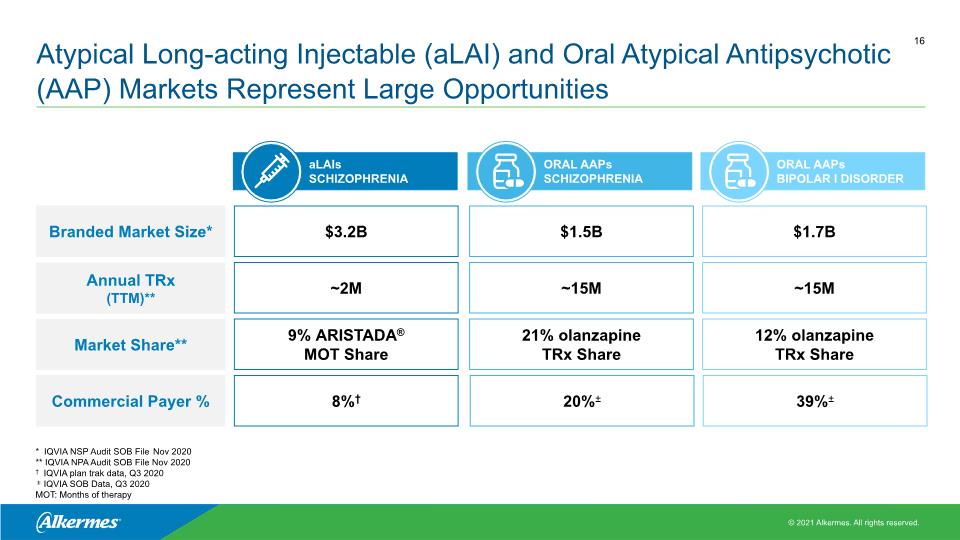
ORAL AAPs BIPOLAR I DISORDER Atypical Long-acting Injectable (aLAI) and Oral Atypical Antipsychotic (AAP) Markets Represent Large Opportunities aLAIs SCHIZOPHRENIA Branded Market Size* Annual TRx (TTM)** Market Share** $3.2B ~2M 9% ARISTADA® MOT Share $1.5B ~15M 21% olanzapine TRx Share $1.7B ~15M 12% olanzapine TRx Share ORAL AAPs SCHIZOPHRENIA * IQVIA NSP Audit SOB File Nov 2020 ** IQVIA NPA Audit SOB File Nov 2020 † IQVIA plan trak data, Q3 2020 ± IQVIA SOB Data, Q3 2020 MOT: Months of therapy Commercial Payer % 8%† 20%± 39%±

LYBALVI™* (ALKS 3831): A Potential New Oral Treatment for Adults With Schizophrenia and Adults With Bipolar I Disorder Daily oral investigational antipsychotic designed to offer efficacy of olanzapine; addition of samidorphan intended to mitigate olanzapine-associated weight gain Fixed-dose combination Bilayer tablet of samidorphan (10 mg) and olanzapine (5 mg, 10 mg, 15 mg, or 20 mg) NDA resubmission under review by FDA; PDUFA date June 1, 2021 ™ *The brand name LYBALVI™ has been conditionally accepted by the FDA and will be confirmed upon approval.

Alkermes Market Research Data Underscores Real-World Unmet Need and Opportunity OLZ is one of the most efficacious oral AAPs for SZ OLZ is the most efficacious oral AAP for maintenance of BD 50% 71% Majority of HCPs Agree OLZ is One of the Most Efficacious Oral AAPs for SZ and BD* Weight Gain is Major Concern to SZ and BD Patients* 73% 82% Weight gain with olanzapine is a major concern to patients Schizophrenia and bipolar disorder patients cycle through 5-7 treatment options** on average ~70K treatment switches occur each month† *Source: ALKS 3831 SZ & BD 2020 ATU; Fielded 2/3 – 3/6/2020; N=124 / 125 HCPs per SZ / BD study. **Source: Market research study; Fielded May-June 2019; 26 BD-I patients, 10 SZ patients, 9 SZ caregivers. † Source: IQVIA SOB data for the oral atypical antipsychotic market, specific to SZ/BD indications. HCPs: healthcare providers; OLZ: olanzapine; AAP: atypical antipsychotic; SZ: schizophrenia; BD: bipolar disorder

LYBALVI™ Focus: Drive Awareness and Plan for Launch in 2021 Launch Planning Awareness Engage in scientific exchange with healthcare providers Continue disease state education campaign for caregivers, providers and thought leaders Conduct investigational product presentations with payers Target well-defined healthcare provider call universe at launch based on firsthand market insights Leverage existing commercial organization Implement patient access programs designed to mitigate payer restrictions early in launch

© 2021 Alkermes. All rights reserved.

Neuroscience Oncology Research and Development Strategy: Focus on High-Value Candidates in Two Key Therapeutic Areas Psychiatry Neurology Engineered Cytokines Small Molecules

Nemvaleukin Alfa (“nemvaleukin”) Formerly referred to as ALKS 4230 Novel investigational immuno-oncology candidate designed to leverage proven anti-tumor effects of interleukin-2 (IL-2) pathway Stable, inherently active, single polypeptide designed to selectively bind to the intermediate-affinity IL-2 receptor complex and expand tumor-killing CD8+ and Natural Killer (NK) T cells, and have negligible effects on Treg expansion

Activation of IL-2 Pathway Offers Broad Potential Clinical Utility IL-2 is a natural regulator of the activity of lymphocytes involved in the immune response Recombinant human IL-2 as monotherapy can drive complete and durable responses in certain tumor types, but its toxicity profile significantly limits its broad use A molecule with differentiated tolerability that targets the IL-2 pathway could be complementary to a wide range of other therapeutic approaches Cancer antigen presentation Vaccines IFN-a Anti-CD40 (agonist) TLR agonists 5 3 2 1 7 6 Infiltration of T cells into tumors CAR-Ts Recognition of cancer cells by T cells CAR-Ts Chemotherapy Radiation Killing of cancer cells Radiation Chemotherapy Targeted Anti-PD-(L)1) Release of cancer cell antigens Chemotherapy Radiation therapy Targeted therapy Priming and activation of Natural Killer and CD8+ T cells IL-2 PD1/L CTLA4 Tumor Lymph node Blood vessel 4 Trafficking of T cells to tumors Anti-VEGF (including TKIs) Range of Potential Therapeutic Combinations with Nemvaleukin Adapted from Chen and Mellman, 2013.

Nemvaleukin Development Strategy CONFIRM mechanism through immune response SEEK anti-tumor activity signals FOCUS on initial registration pathways BROADEN program to maximize value

Nemvaleukin Development Strategy Demonstrate clinical pharmacodynamic response reflective of intermediate-affinity IL-2 receptor selectivity Dose-dependent, selective expansion of NK and CD8+ T cells Minimal and non-dose dependent changes in peripheral regulatory T cells Advance into phase 2 expansion stage of ARTISTRY-1 and ARTISTRY-2 Additional phase 1 pharmacokinetic/pharmacodynamic, safety, and tolerability data for subcutaneous nemvaleukin to be submitted to upcoming medical meeting CONFIRM mechanism through immune response SEEK anti-tumor activity signals FOCUS on initial registration pathways BROADEN program to maximize value

Nemvaleukin Development Strategy Demonstrate monotherapy anti-tumor efficacy Single-agent activity observed in mucosal melanoma and renal cell carcinoma with IV nemvaleukin Demonstrate anti-tumor activity in combination with PD-1 inhibitor Durable and deepening responses observed with IV nemvaleukin in combination with pembrolizumab Responses in multiple solid tumor types (melanoma, ovarian, breast, cervical, gastro-esophageal and pancreatic) with IV nemvaleukin CONFIRM mechanism through immune response SEEK anti-tumor activity signals FOCUS on initial registration pathways BROADEN program to maximize value
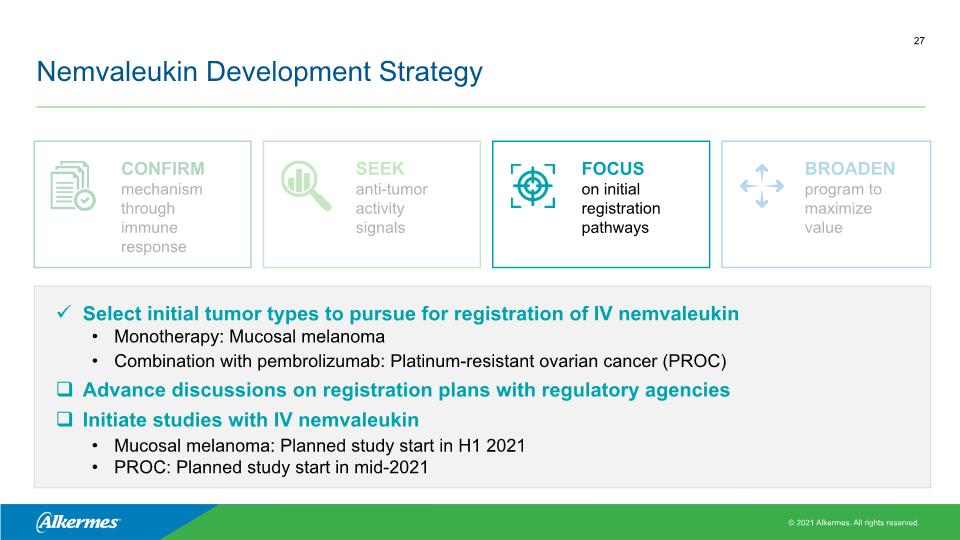
Nemvaleukin Development Strategy Select initial tumor types to pursue for registration of IV nemvaleukin Monotherapy: Mucosal melanoma Combination with pembrolizumab: Platinum-resistant ovarian cancer (PROC) Advance discussions on registration plans with regulatory agencies Initiate studies with IV nemvaleukin Mucosal melanoma: Planned study start in H1 2021 PROC: Planned study start in mid-2021 CONFIRM mechanism through immune response SEEK anti-tumor activity signals FOCUS on initial registration pathways BROADEN program to maximize value

Nemvaleukin Development Strategy Identify and select additional tumor types and combinations to pursue Earlier lines of therapy versus standard of care Combinations with targeted and immuno-oncology agents Combinations with radiotherapy, chemotherapy, or other therapies Strategic collaboration CONFIRM mechanism through immune response SEEK anti-tumor activity signals FOCUS on initial registration pathways BROADEN program to maximize value

ALKS 1140: Novel CoREST-Selective HDAC Inhibitor for Neuropsychiatric Indications First candidate nominated from platform of selective HDAC inhibitor compounds Inhibition of the CoREST HDAC complex is a novel approach to increase functional synaptic connections and synaptic integrity Initial clinical development plans focused on basket of indications, including rare neurodegenerative and neurodevelopmental diseases as well as common psychiatric diseases HDAC: Histone deacetylase CoREST: Co-repressor of repressor element-1 silencing transcription factor
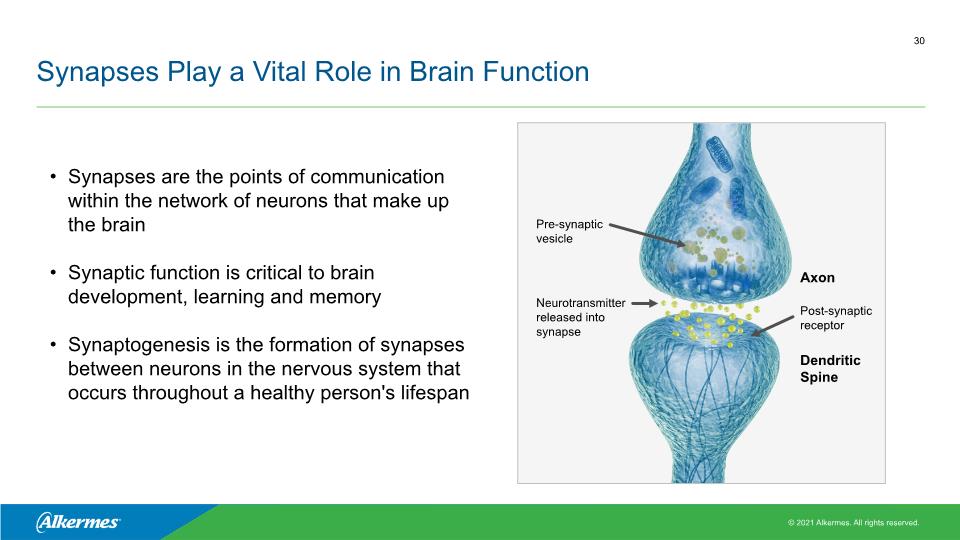
Synapses Play a Vital Role in Brain Function Synapses are the points of communication within the network of neurons that make up the brain Synaptic function is critical to brain development, learning and memory Synaptogenesis is the formation of synapses between neurons in the nervous system that occurs throughout a healthy person's lifespan

Many neurological disorders are characterized by synaptic pathology Abnormal density of dendritic spines Synapse loss Aberrant synaptic signaling and plasticity Synapses are a critical target to slow progression and preserve cognitive and functional abilities in multiple neuropsychiatric, neurodegenerative and neurodevelopment disorders Synaptic Loss: Pathologic Correlate of Cognitive Decline Onwordi et al, Nature Communications. 11, 246 (2020). https://www.nature.com/articles/s41467-019-14122-0#citeas Impaired Brain Function Healthy Control

ALKS 1140 Selectively Modulates the Function of the CoREST HDAC Complex Criteria achieved for nomination of ALKS 1140 as a candidate to improve synapse formation and synapse function: Selectively inhibits HDAC1/2 specifically associated with the CoREST complex Brain permeable Designed to avoid hematopoietic side effects Increased synaptic density in preclinical models Plan to begin first-in-human trials in 2021 LSD1, HDAC1, CoREST1 and ALKS 1140 modeled into the small angle X-ray scattering (SAXS) envelope of the CoREST complex (Cell Reports 30:2699, 2020)

Upcoming Q1 Investor Day: Focus on the Pipeline Development candidates Nemvaleukin clinical development update ALKS 1140 background and development plan Scientific platforms Engineered cytokines HDAC inhibitors Small molecule medicinal chemistry Therapeutic area focus Psychiatry Neurology Oncology

Focus on Value Creation in 2021 Grow and Diversify Commercial Revenues Demonstrate Value of R&D Investments Manage for Growth & Long-Term Profitability

www.alkermes.com
