UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
ALKERMES PUBLIC LIMITED COMPANY
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
||
|
(State or other jurisdiction |
|
(Commission |
|
(IRS Employer |
||
|
of incorporation) |
|
File Number) |
|
Identification No.) |
||
|
|
|
|
||||
|
|
|
|
||||
|
|
||||||
|
|
||||||
|
(Address of principal executive offices) |
||||||
Registrant's telephone number, including area code: +
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 9, 2023, Alkermes plc made available a copy of the corporate presentation to be displayed during its presentation at the J.P. Morgan Healthcare Conference on January 11, 2023. A copy of the presentation is furnished herewith as Exhibit 99.1 and is incorporated by reference in this Item 7.01.
The information in this Item 7.01, and in Exhibit 99.1 furnished herewith, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit |
|
|
|
No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
ALKERMES PLC |
||
|
|
|
||
|
Date: January 9, 2023 |
By: |
|
/s/ David J. Gaffin |
|
|
|
|
David J. Gaffin |
|
|
|
|
Secretary |
3

Richard Pops Chief Executive Officer A Clear Investment Thesis January 2023 41st Annual J.P. Morgan Healthcare Conference Exhibit 99.1

Forward-Looking Statements Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the company’s expectations with respect to its current and future financial and operating performance, business plans or prospects, including its expected value drivers and growth opportunities and expectations concerning profitability; the company’s plans to separate its neuroscience and oncology businesses, including the anticipated benefits of a potential separation, the anticipated elements and value propositions of the two standalone businesses and their ability to attract investors; the potential therapeutic and commercial value of the company’s marketed products and development candidates, including nemvaleukin alfa (“nemvaleukin”) as a cancer immunotherapy when used as monotherapy or in combination, its potential clinical utility across a range of tumor types, dosing options and potential combinations, the company’s orexin 2 receptor agonist program for narcolepsy and the company’s platform of early-stage engineered cytokines; timelines, plans and expectations for development activities relating to the company’s development candidates, including for ALKS 2680, plans for its first-in-human studies and potential for clinical proof-of-concept data; the company’s commercial activities, including its plans to launch a direct-to-consumer advertising campaign for LYBALVI. The company cautions that forward-looking statements are inherently uncertain. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: the company may not ultimately separate its oncology business in a timely manner or at all; unanticipated developments, costs or difficulties that may delay or otherwise negatively affect the planned separation of the company’s neuroscience and oncology businesses or the anticipated benefits of such separation; the impacts of the ongoing COVID-19 pandemic and continued efforts to mitigate its spread on the company’s business, results of operations or financial condition; the unfavorable outcome of arbitration or litigation and so-called “Paragraph IV” litigation or other patent litigation which may lead to competition from generic drug manufacturers, or other disputes related to the company’s products or products using the company’s proprietary technologies; planned commercial activities may not be launched on the anticipated timelines or result in the benefits that the company anticipates; clinical development activities may not be completed on time or at all; the results of the company’s development activities may not be positive, or predictive of final results from such activities, results of future development activities or real-world results; the U.S. Food and Drug Administration (“FDA”) or other regulatory authorities may not agree with the company’s regulatory approval strategies or components of the company’s marketing applications and may make adverse decisions regarding the company’s products; the company and its licensees may not be able to successfully commercialize their products or support growth from such products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to government payers; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended Dec. 31, 2021 and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), including the company's Quarterly Report on Form 10-Q for the quarter ended Sept. 30, 2022, which are available on the SEC’s website at www.sec.gov, and on the company’s website at www.alkermes.com in the “Investors – SEC filings” section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Note Regarding Trademarks: The company and its affiliates are the owners of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, VIVITROL®, and LYBALVI®. VUMERITY® is a registered trademark of Biogen MA Inc., used by Alkermes under license. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto.

Alkermes Today Proprietary Commercial Products Established Commercial Infrastructure in Complex Markets Royalty & Manufacturing Revenues* Complex Manufacturing Capabilities Neuroscience Development Pipeline Oncology Development Pipeline Neuroscience and Oncology R&D Capabilities *VUMERITY is licensed to and commercialized exclusively by Biogen

Post-Separation: Clear Value Propositions in Neuroscience and Oncology Proprietary Commercial Products Established Commercial Infrastructure in Complex Markets Complex Manufacturing Capabilities Neuroscience R&D Capabilities and Pipeline Oncology Development Pipeline & Capabilities Oncology Company** *VUMERITY is licensed to and commercialized exclusively by Biogen **Assuming separation of the company’s oncology business is effected through a spin-off of the oncology business into an independent, publicly-traded company Royalty & Manufacturing Revenues*

Clear Priorities to Unlock Value in 2023 2023 Business Priorities and Value Drivers Drive launch of LYBALVI® Continued execution of commercial strategy and investment in DTC campaign Advance orexin 2 receptor agonist Establish initial safety and tolerability profile and generate initial clinical proof-of-concept data for ALKS 2680 Separate oncology business Clarify value proposition for standalone neuroscience and oncology businesses DTC: Direct-to-consumer

Drive Launch of LYBALVI®

LYBALVI® (olanzapine and samidorphan): Oral Treatment Option for Adults With Schizophrenia or Bipolar I Disorder Once-daily, oral atypical antipsychotic composed of olanzapine, an established antipsychotic agent, and samidorphan, a new chemical entity Commercially launched in U.S. Q4 2021 with differentiated label Indicated for the treatment of: Schizophrenia in adults Bipolar I disorder in adults Acute treatment of manic or mixed episodes as monotherapy and as adjunct to lithium or valproate Maintenance monotherapy treatment Boxed Warning: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. LYBALVI is not approved for the treatment of patients with dementia-related psychosis. Full prescribing information may be found at www.lybalvi.com/lybalvi-prescribing-information.pdf
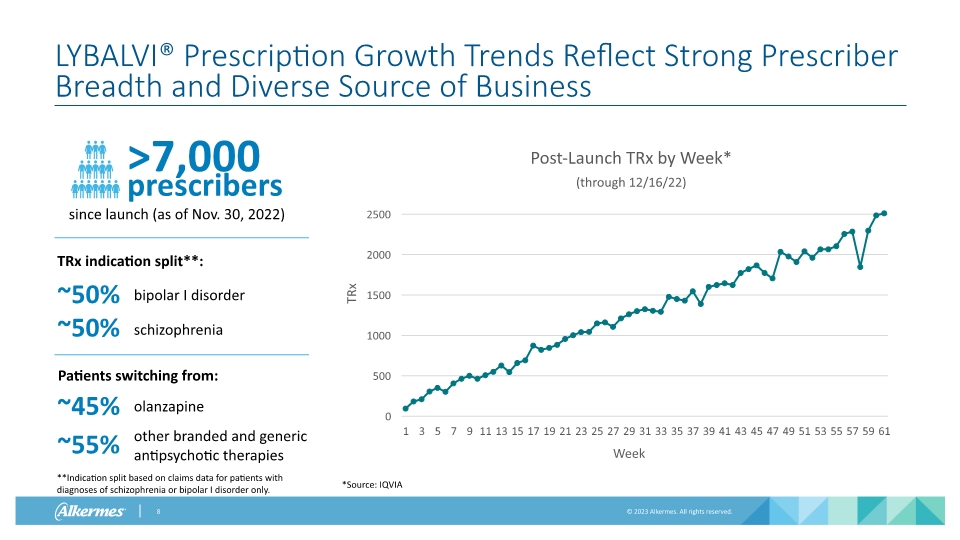
LYBALVI® Prescription Growth Trends Reflect Strong Prescriber Breadth and Diverse Source of Business TRx Week >7,000 prescribers since launch (as of Nov. 30, 2022) bipolar I disorder ~50% other branded and generic antipsychotic therapies olanzapine ~50% schizophrenia ~45% ~55% TRx indication split**: Patients switching from: *Source: IQVIA **Indication split based on claims data for patients with diagnoses of schizophrenia or bipolar I disorder only.
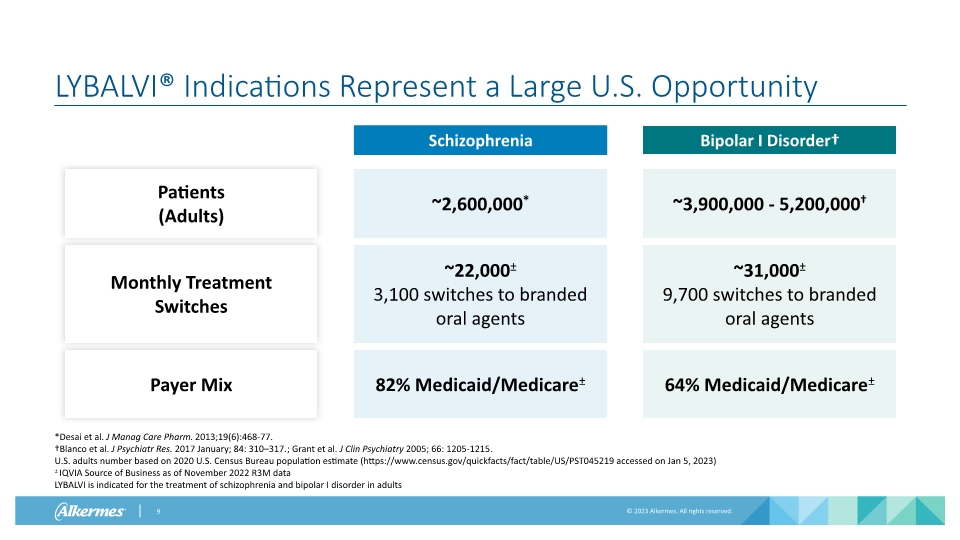
LYBALVI® Indications Represent a Large U.S. Opportunity Schizophrenia Bipolar I Disorder† Patients (Adults) Monthly Treatment Switches ~2,600,000* ~22,000± 3,100 switches to branded oral agents ~3,900,000 - 5,200,000† ~31,000± 9,700 switches to branded oral agents *Desai et al. J Manag Care Pharm. 2013;19(6):468-77. †Blanco et al. J Psychiatr Res. 2017 January; 84: 310–317.; Grant et al. J Clin Psychiatry 2005; 66: 1205-1215. U.S. adults number based on 2020 U.S. Census Bureau population estimate (https://www.census.gov/quickfacts/fact/table/US/PST045219 accessed on Jan 5, 2023) ± IQVIA Source of Business as of November 2022 R3M data LYBALVI is indicated for the treatment of schizophrenia and bipolar I disorder in adults Payer Mix 82% Medicaid/Medicare± 64% Medicaid/Medicare±
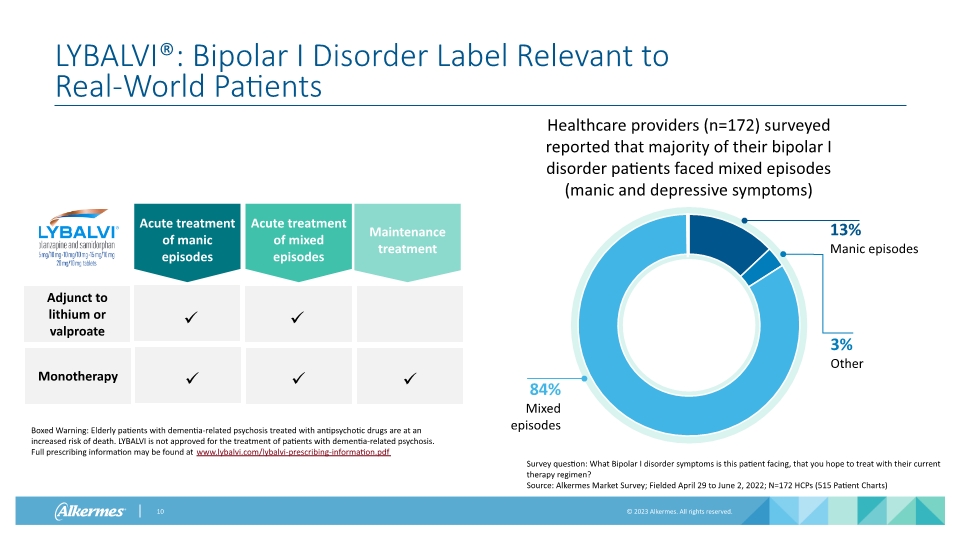
LYBALVI®: Bipolar I Disorder Label Relevant to Real-World Patients Survey question: What Bipolar I disorder symptoms is this patient facing, that you hope to treat with their current therapy regimen? Source: Alkermes Market Survey; Fielded April 29 to June 2, 2022; N=172 HCPs (515 Patient Charts) Healthcare providers (n=172) surveyed reported that majority of their bipolar I disorder patients faced mixed episodes (manic and depressive symptoms) Acute treatment of manic episodes Acute treatment of mixed episodes Maintenance treatment Adjunct to lithium or valproate Monotherapy Boxed Warning: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. LYBALVI is not approved for the treatment of patients with dementia-related psychosis. Full prescribing information may be found at www.lybalvi.com/lybalvi-prescribing-information.pdf

Plans to Launch LYBALVI® Direct-to-Consumer Advertising Campaign Focused on Bipolar I Disorder Boxed Warning: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. LYBALVI is not approved for the treatment of patients with dementia-related psychosis. Full prescribing information may be found at www.lybalvi.com/lybalvi-prescribing-information.pdf

LYBALVI®: Strong Launch Trajectory Established in 2022 With Clear Strategic Focus in 2023 LYBALVI Quarterly Net Sales ($M) 2023 Commercial Strategy Focus Breadth: Continue to drive prescriber breadth through highly-targeted field force deployment Access: Expand patient access through continued execution of disciplined payer contracting strategy Awareness: Launch DTC advertising campaign focused on digital and broadcast channels

Advance Orexin 2 Receptor (OX2R) Agonist

Orexin Dysfunction: Well Defined Opportunity in Narcolepsy and Other Sleep Disorders In narcolepsy and other sleep disorders, low orexin levels lead to inconsistent neurotransmitter release, resulting in excessive sleepiness and poor regulation of REM sleep Narcolepsy affects ~200,000 people in U.S. and 3M people globally1 70% of people with narcolepsy have narcolepsy type 12, distinguished by: Cataplexy, a sudden muscle weakness triggered by strong emotions Low or no orexin in the brain Genetic and pharmacologic evidence suggests that orexin receptor agonists, especially OX2R agonists, may be useful for mechanistic therapy of narcolepsy3 Figure from: Scammell, T E, and Saper, C B. Nature medicine. 2007;13:126-8 1 Global Narcolepsy Drugs Market, Forecast 2019-2025. Allied Market Research 2 Swick TJ. Treatment paradigms for cataplexy in narcolepsy: past, present, and future. Nat Sci Sleep. 2015;7:159-169 3 Nagahara T. Design and Synthesis of Non-Peptide, Selective Orexin Receptor 2 Agonists. J. Med. Chem. 2015;58:7931–7937

Leveraging Alkermes’ Molecular Design Capabilities to Target Orexin Dysfunction ALKS 2680 molecular design objectives: Capture performance of endogenous peptide OX2R agonist Increased wakefulness duration Improved cataplexy control Optimize potency and minimize predicted human dose to mitigate risk of undesired side effects Provide PK/PD profile that mirrors natural wake cycle Dose to allow for 8-12 hours wakefulness without subsequent insomnia Enable convenient once-daily, oral medication PK: pharmacokinetic; PD: pharmacodynamic Orexin 2 Receptor in Complex with Peptide-Agonist Orexin-B Figure adapted from: Hong, Chuan, et al. Nature communications. 2021:12; 3. PDB ID: 7L1U
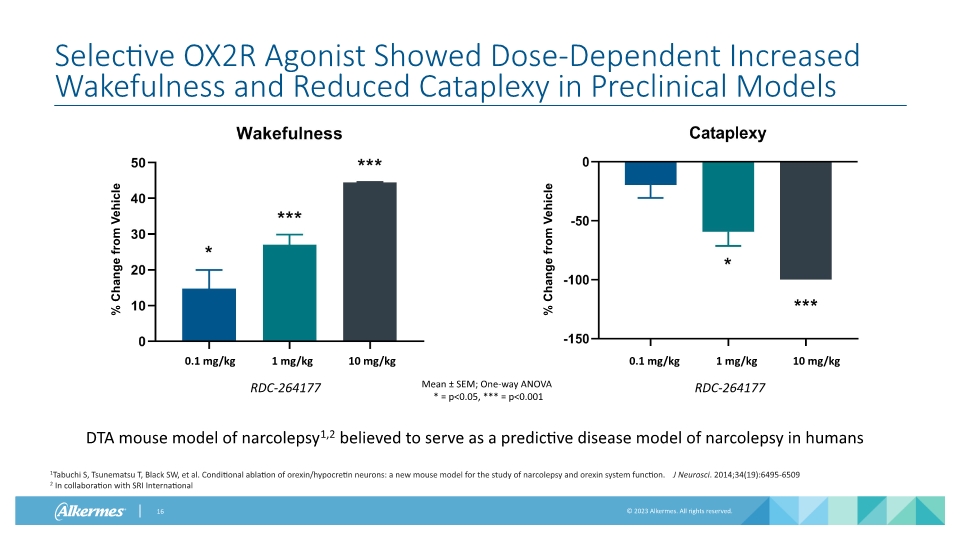
Selective OX2R Agonist Showed Dose-Dependent Increased Wakefulness and Reduced Cataplexy in Preclinical Models Mean ± SEM; One-way ANOVA * = p<0.05, *** = p<0.001 0.1 mg/kg 1 mg/kg 10 mg/kg RDC-264177 0.1 mg/kg 1 mg/kg 10 mg/kg RDC-264177 DTA mouse model of narcolepsy1,2 believed to serve as a predictive disease model of narcolepsy in humans % Change from Vehicle % Change from Vehicle 1Tabuchi S, Tsunematsu T, Black SW, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495-6509 2 In collaboration with SRI International

Selective Orexin 2 Receptor Agonist Promoted Prolonged Wakefulness in Preclinical In Vivo Studies Selective orexin 2 receptor agonist demonstrated dose-dependent wake duration (shown by red gamma bands and dark blue theta and delta bands) PharmacoEEG: Pharmaco-electroencephalography Alkermes data on file
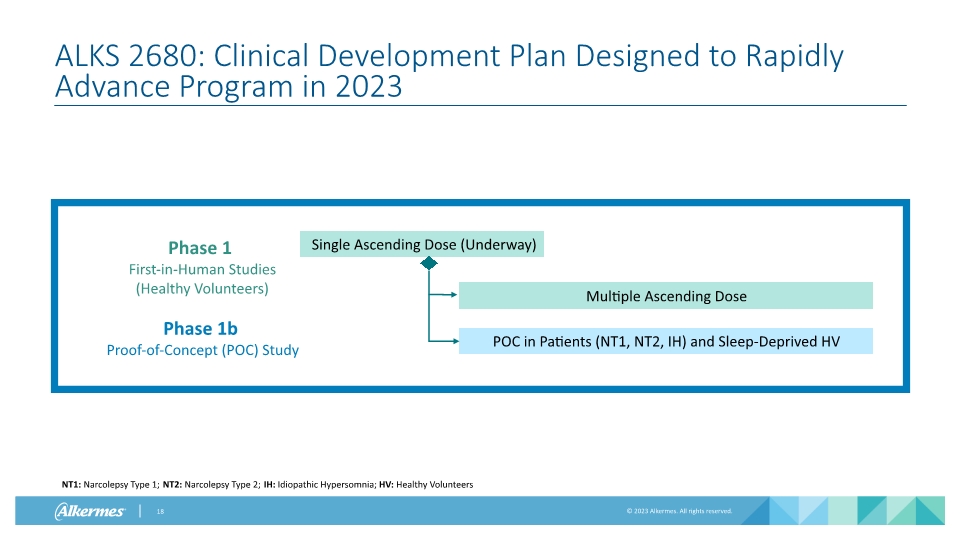
Single Ascending Dose (Underway) Multiple Ascending Dose POC in Patients (NT1, NT2, IH) and Sleep-Deprived HV ALKS 2680: Clinical Development Plan Designed to Rapidly Advance Program in 2023 Phase 1 First-in-Human Studies (Healthy Volunteers) Phase 1b Proof-of-Concept (POC) Study NT1: Narcolepsy Type 1; NT2: Narcolepsy Type 2; IH: Idiopathic Hypersomnia; HV: Healthy Volunteers

ALKS 2680 Clinical Development Plan Targeting Clinical Proof-of-Concept Data by Year-End 2023 Phase 1b Proof-of-Concept (POC) Study Objective: Early POC data + dose range estimation for phase 2 in lead indications Key Assessments: EEG-based maintenance of wakefulness test as primary efficacy/PD readout Drug exposure (PK) Evaluate safety and tolerability Study initiation expected H1 2023; Preliminary data expected by year-end Phase 1 First-in-Human (FIH) Studies Objective: Measure and model pharmacokinetics (PK)/ pharmacodynamics (PD) and evaluate safety and tolerability of single and multiple ascending doses Key Assessments: Drug exposure (PK) Evaluate safety and tolerability of single and multiple ascending doses Exploratory assessment of target engagement: qEEG trends in power of frequency bands Single-ascending dose study ongoing; Multiple-ascending dose study initiation expected Q1 2023 EEG: electroencephalogram; qEEG: quantitative electroencephalogram

Separate Oncology Business

Post-Separation Oncology Co. Pure-Play, Development-Stage Oncology Company Investment thesis anchored by potential medical and economic value of nemvaleukin alfa: Potential first-in-class IL-2 variant immunotherapy Anti-tumor activity observed both as a single agent and with checkpoint inhibitors (CPI), in CPI-unapproved tumor types and post-CPI settings Potential registration-enabling studies underway in mucosal melanoma* and platinum-resistant ovarian cancer**, each with FDA Fast Track Designation Investigating alternative routes of administration/dosing schedules *Also granted FDA Orphan Drug Designation; **In combination with pembrolizumab Assuming separation is effected through a spin-off of the oncology business into an independent, publicly-traded company Sophisticated protein engineering platform capabilities and early-stage development assets Tumor-targeted split IL-12 program IL-18 program Nemvaleukin offers an opportunity for significant value creation as the development program advances and expands Highly-experienced team with scientific and clinical trial expertise to efficiently advance pipeline Opportunity to attract oncology-focused investors

ARTISTRY-1 (IV Nemvaleukin): Durable Responses Observed Demonstrated durable responses in high unmet need populations Monotherapy activity (IV) in prior anti-PD-(L)1 treated melanoma and renal cell carcinoma Combination activity (IV) with pembrolizumab in a range of tumor types Treatment-related adverse events (AEs) have been consistent with expectations based on nemvaleukin’s mechanism of action and were mostly transient and manageable Pyrexia, chills and nausea were the most commonly reported AEs; Transient and asymptomatic neutropenia/neutrophil count decrease were the most commonly reported events of grade ≥3 Three dose-limiting toxicities were reported, all in the highest dose evaluated (declared as the maximum tolerated dose) Patients achieved SD (*acral), PR (+RCC), and PD (#RCC) on nemvaleukin monotherapy, rolled over to combination therapy and achieved PR. IV: Intravenous; NSCLC: Non-small cell lung cancer; SCLC: Small cell lung cancer; RCC: Renal cell carcinoma; SCC: Squamous cell carcinoma CR: complete response; PR: partial response; uPR: unconfirmed PR Monotherapy and Combination Responses Data as of August 2022

Nemvaleukin ARTISTRY Development Program ARTISTRY-6 Status IDMC: Risk/benefit profile supports study continuation as planned Mucosal melanoma enrollment ongoing Cutaneous cohort (SC) enrollment nearing completion Plan to initiate POC cohort with LFIV dosing (cutaneous) Status Enrollment closed Data maturing, focus on durability of responses compared to daily IVx5 Data from ARTISTRY-2 and ARTISTRY-6 will inform viability of SC Status Site initiation and enrollment ongoing In collaboration with MSD In partnership with the GOG Foundation and ENGOT Status Enrolling Day 1 Q3W and Day 1,4 Q3W dosing cohorts at pharmacologically relevant doses Escalation ongoing IV: Intravenous; LFIV: Less frequent IV; SC: Subcutaneous; IDMC: Independent Data Monitoring Committee; MSD: A tradename of Merck & Co., Inc. Kenilworth, NJ, USA; ENGOT: European Network of Gynaecological Oncological Trial Groups Advanced cutaneous and mucosal melanoma Phase 2 Dosing: Daily IVx5, SC Q1W Monotherapy Potential Registration Enabling Studies Alternative Dosing Studies Platinum-resistant ovarian cancer Phase 3 Dosing: Daily IVx5 Combination with pembrolizumab ARTISTRY-7 ARTISTRY-2 Advanced solid tumors Phase 1/2 Dosing: SC Q1W Combination with pembrolizumab Advanced solid tumors Phase 1/2 Dosing: LFIV dose escalation Monotherapy and combination with pembrolizumab ARTISTRY-3

Positioning Alkermes Neuroscience Business for Future Growth

Post-Separation Alkermes* Pure-Play, Commercial-Stage Neuroscience Company Separation expected to enhance profitability Builds on Alkermes’ innovation and excellence in neuroscience *Assuming separation of the company’s oncology business is effected through a spin-off of the oncology business into an independent, publicly-traded company Proprietary Products Topline primarily driven by growth of proprietary commercial products in addiction and psychiatry Complex manufacturing capabilities Development Pipeline Early-stage neuroscience pipeline ALKS 2680, orexin 2 receptor agonist in phase 1 Portfolio of preclinical neuroscience assets Commercial Capabilities Established commercial capabilities in complex psychiatry and addiction markets Opportunity to capture further operating leverage

Topline Growth and Diversification Reflect Evolving Business *Inclusive of ARISTADA INITIO® **Licensed product (royalty & manufacturing revenue) Plan to provide 2023 financial expectations and updated long-term profitability targets in Q1’23

Clear Priorities to Unlock Value in 2023 2023 Business Priorities and Value Drivers Drive launch of LYBALVI® Continued execution of commercial strategy and investment in DTC campaign Advance orexin 2 receptor agonist Establish initial safety and tolerability profile and generate initial clinical proof-of-concept data for ALKS 2680 Separate oncology business Clarify value proposition for standalone neuroscience and oncology businesses DTC: Direct-to-consumer

www.alkermes.com
